Introduction
The Ki-67 index has been previously shown to be prognostic, wherein the cut-off of 30% was associated with inferior survival outcomes (Determann O et al, Blood 2008) in newly diagnosed MCL patients (pts). However, this cut-off determination was made in pts treated with CHOP/R-CHOP as the first-line treatment, controlled only for MIPI, and predates the advent of novel agents. Short diagnosis to treatment interval (DTI ≤ 14 days) was recently shown to be prognostic in newly diagnosed MCL but its association with Ki-67 is unknown. We sought to determine the optimal Ki-67 cut-off in MCL and its prognostic relevance in the modern era.
Methods
This is a pooled analysis of 2 large datasets, one prospective (CALGB 50403) and one retrospective (MCL real world study [MCL-RWS]). CALGB is now part of the Alliance for Clinical Trials in Oncology. CALGB 50403 is a phase II randomized study wherein newly diagnosed MCL pts received intensive induction chemoimmunotherapy (IIC) followed by autologous transplant, and posttransplant rituximab. The MCL-RWS included adult pts (≥18 years [yrs]) with MCL treated from 2000 to 2017 at 12 participating US sites. Pts from sites in the MCL-RWS that enrolled on CALGB 50403 were excluded.
Ki-67 was scored by central review in 10% increments in the CALGB 50403 and per institutional standard in the MCL-RWS.
We assessed Ki-67 as a continuous variable and used a restricted quadratic spline fit to find an optimal cut-off. We looked at the associations between Ki-67 and other known/ established MCL prognostic markers including MIPI and DTI. The outcome parameters were PFS and OS. Time-to-event endpoints were evaluated by Kaplan-Meier and Cox proportional hazard methods.
Results
Among the 793 pts (n=666, MCL-RWS and n=127, CALGB 50403) in the pooled analysis, Ki-67 was available in 385 pts (311 from MCL-RWS and 74 from CALGB 50403). The median OS (mOS) and PFS (mPFS) in the entire cohort (n=793) was 11.8 years and 4.7 years, respectively. The two groups (gps), those with and without Ki-67 data, were well balanced including MIPI and DTI.
The median age was 62 yrs (range, 32-88), 81% males, 94% with ECOG performance status (PS) 0-1 and 84% with stage 4.
We found a Ki-67 cut-off of 50% to be optimal as the relative hazard of death rises after 50%. Pts in the Ki-67 >50% gp had significantly higher proportion with short DTI (36% vs 17%, p<0.001), ECOG PS ≥2 (12% vs 5%, p=0.02), elevated LDH (58% vs 39%, p=0.005), and MIPI ≥6.2 (38% vs 20%, p=0.007) compared to Ki-67 of ≤50%.
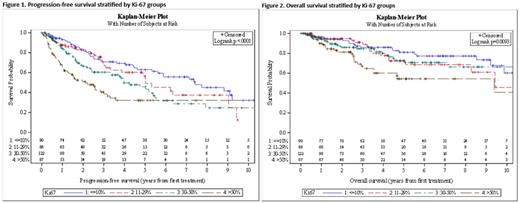
Ki-67 >50% was associated with significantly inferior PFS with a hazard ratio (HR) of 2.10 (95%CI 1.50-2.92) compared to Ki-67 ≤50% with a mPFS of 2.1 vs 5.3 yrs, respectively, regardless of the age (young [≤65 yrs], 2.6 vs 5.3 yrs or old [>65 yrs], 1.1 vs 5.4 yrs) or receipt of IIC (2.2 vs 5.9 yrs). Figure 1 shows the statistically significant difference in PFS between the Ki-67 gps of ≤10%, >10% to <30%, ≥30 to ≤50%, and >50%. Ki-67 >50% remained prognostic for PFS in multivariable analysis (MVA) after adjusting for significant variables in univariable analysis (UVA) including short DTI, ECOG PS ≥2, stage 4, elevated LDH, and MIPI ≥6.2 with adjusted HR (aHR) 2.19, 95%CI 1.38-3.48, p <0.001.
Ki-67 >50% was associated with significantly inferior OS with a HR of 2.10 (95%CI 1.33-3.32) compared to Ki-67 ≤50% with a mOS of 9.4 yrs vs not reached (NR), respectively, regardless of the age or receipt of IIC. Figure 2 shows the statistically significant difference in OS between the Ki-67 gps of ≤10%, >10% to <30%, ≥30 to ≤50%, and >50%. Ki-67 >50% was not prognostic for OS in MVA after adjusting for significant variables in the UVA (MIPI and sex), aHR 1.79, 95% CI 0.92-3.49, p=0.08. Similar findings were seen in multivariable analysis with Ki-67 >30%, which showed no prognostic significance (aHR 1.46, 95%CI 0.82-2.58, p=0.19).
Because of disparate datasets, we performed sensitivity analysis looking at the prognostic relevance of in individual datasets (CALGB 50403 and MCL-RWS) and found Ki67 >50% was prognostic for PFS and OS in both of the datasets consistent with the primary analysis.
Conclusions
This study evaluating the optimal Ki-67 cut off for pts with newly diagnosed MCL in the modern era found that Ki-67 > 50% was prognostic for PFS but not OS in MVA. A potential reason for the lack of prognostic relevance for OS in this more recent dataset is the availability of effective options at relapse. We also found that short DTI was associated with high Ki-67 gp which is a novel finding.
Disclosures
Epperla:ADC Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Speakers Bureau; Beigene: Research Funding, Speakers Bureau; Lilly: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Bachanova:Gamida Cell: Research Funding; Incyte: Research Funding; Citius: Research Funding; BMS: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; ADC: Membership on an entity's Board of Directors or advisory committees; Allogene: Membership on an entity's Board of Directors or advisory committees; Miltenyi: Other: DSMB. Barta:Acrotech: Consultancy; Janssen: Consultancy; Affimed: Consultancy; Daiichi Sankyo: Consultancy. Danilov:Beigene: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Bayer: Research Funding; MEI: Consultancy, Research Funding; Lilly Oncology: Consultancy, Research Funding; Nurix: Consultancy, Research Funding; Cyclacel: Research Funding; Janssen: Consultancy; Astra Zeneca: Consultancy, Research Funding; Bristol Meyers Squibb: Consultancy, Research Funding; Genentech: Consultancy; GenMab: Consultancy, Research Funding; Merck: Consultancy. Grover:ADC Therapeutics: Consultancy, Honoraria; Kite: Honoraria; Genentech: Honoraria; Seagen: Honoraria; Caribou Biosciences: Honoraria; Tessa Therapeutics: Research Funding; Novartis: Honoraria; Sangamo: Current holder of stock options in a privately-held company; Seattle Genetics: Consultancy. Karmali:Takeda: Research Funding; Kite/Gilead: Consultancy, Honoraria, Research Funding; Miltenyi: Consultancy, Honoraria, Research Funding; Genentech/Roche: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; Lilly: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding; Calithera: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Morphosys: Consultancy, Speakers Bureau; Janssen: Consultancy. Hill:BeiGene: Consultancy; Bristol Myers Squibb: Consultancy; Genentech: Consultancy, Other: Advisory board, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Other: travel support, Research Funding; Pharmacyclics: Consultancy, Other: Advisory board, Research Funding; Incyte: Consultancy; Gilead: Other: Advisory board; AstraZeneca: Consultancy; AbbVie: Consultancy, Other: Advisory board, Research Funding. Ghosh:AstraZeneca, Janssen, Pharmacyclics, Kite pharma, BMS, Epizyme: Speakers Bureau; TG Therapeutics, Genentech/Roche, Bristol Myers Squibb, Gilead, Morphosys, AbbVie, Pharmacyclics: Research Funding; Seagen, TG Therapeutics, AstraZeneca, Phamacyclics, Janssen, Bristol Myers Squibb, Gilead Sciences, Kite Pharma, Beigene, Incyte, Lava Therapeutics, Incyte, Roche/Genentech, Novartis, Loxo Oncology, AbbVie, Genmab, Adaptive Biotech, ADC Therapeutics, Morp: Honoraria; Seagen, TG Therapeutics, AstraZeneca, Phamacyclics, Janssen, Bristol Myers Squibb, Gilead Sciences, Kite Pharma, Beigene, Incyte, Lava Therapeutics, Incyte, Roche/Genentech, Novartis, Loxo Oncology, AbbVie, Genmab, Adaptive Biotech, ADC Therapeutics: Consultancy; Roche NHL solutions panel: Membership on an entity's Board of Directors or advisory committees. Fenske:TG Therapeutics: Consultancy, Speakers Bureau; Servier Pharmaceuticals: Consultancy, Speakers Bureau; SeaGen: Consultancy, Speakers Bureau; Sanofi: Consultancy, Speakers Bureau; Pharmacyclics (AbbVie): Consultancy, Speakers Bureau; MorphoSys: Consultancy, Speakers Bureau; Kite (Gilead): Consultancy, Speakers Bureau; Astrazeneca: Consultancy, Speakers Bureau; Beigene: Consultancy, Speakers Bureau; Adaptive Biotechnologies: Consultancy, Speakers Bureau. Martin:AbbVie, AstraZeneca, Beigene, Epizyme, Genentech, Gilead, Janssen, Pepromene, Daiichi Sankyo: Consultancy. Flowers:Morphosys: Research Funding; Novartis: Research Funding; Ziopharm: Research Funding; Burroghs Wellcome Fund: Research Funding; Eastern Cooperative Oncology Group: Research Funding; Kite: Research Funding; Jannsen Pharmaceuticals: Research Funding; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Denovo Biopharma: Consultancy; Allogene: Research Funding; Bayer: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Acerta: Research Funding; Gilead: Consultancy, Research Funding; Karyopharm: Consultancy; Pharmacyclics Jansen: Consultancy; Spectrum: Consultancy; Beigene: Consultancy; N-Power Medicine: Consultancy, Current holder of stock options in a privately-held company; Amgen: Research Funding; Cellectis: Research Funding; Pfizer: Research Funding; Pharmacyclics: Research Funding; Sanofi: Research Funding; Takeda: Research Funding; TG Therapeutics: Research Funding; Xencor: Research Funding; SeaGen: Consultancy; Guardant: Research Funding; Iovance: Research Funding; 4D: Research Funding; Adaptimmune: Research Funding; National Cancer Institute: Research Funding; V Foundation: Research Funding; Cancer Prevention and Research Institute of Texas: Research Funding; CPRIT Scholar in Cancer Research: Research Funding; Abbvie: Consultancy, Research Funding; Genentech Roche: Consultancy, Research Funding; Nektar: Research Funding; Genmab: Consultancy. Hsi:Novartis: Consultancy. Maddocks:Merck: Research Funding; Novartis: Research Funding; Seattle Genetics: Consultancy; Eli Lilly and Company: Consultancy; Epizyme: Consultancy; BeiGene: Consultancy; Gilead/Kite: Consultancy; Pharmacyclics: Consultancy, Research Funding; Morphosys: Consultancy; Janssen: Consultancy, Honoraria; GenMab: Consultancy; Genentech: Consultancy; Incyte: Consultancy, Honoraria; ADC Therapeutics: Consultancy; Celgene: Consultancy, Research Funding; BMS: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; AbbVie: Consultancy. Blum:Cullinan Oncology, Inc.: Research Funding; Seattle Genetics: Research Funding; BMS: Research Funding. Habermann:BMS: Research Funding; Genentech: Research Funding; sorrento: Research Funding. Maurer:Roche/Genentech: Research Funding; BMS: Consultancy, Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees. Bartlett:ADC Therapeutics, Foresight Diagnostics, Kite, F. Hoffmann-La Roche Ltd / Genentech, Inc., Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; Washington University School of Medicine: Current Employment; ADC Therapeutics, Autolus, BMS/Celgene, Forty Seven, Gilead/Kite Pharma, Janssen, Merck, Millennium, Pharmacyclics, F. Hoffmann-La Roche Ltd / Genentech, Inc., Seattle Genetics: Research Funding. Leonard:National Cancer Institute, Leukemia and Lymphoma Society, Genentech, Epizyme, Janssen: Research Funding; AbbVie, AstraZeneca, Astellas, Bayer, BeiGene, BMS, Calithera, Constellation, Eisai, Epizyme, GenMab, Grail, Incyte, Janssen, Karyopharm, Lilly, Merck, Mustang Bio, Pfizer, Roche/Genentech, Seagen, Second Genome, Sutro: Consultancy. Cohen:Novartis: Research Funding; BMS/Celgene: Research Funding; Genentech: Research Funding; BioInvent: Research Funding; Lam Therapeutics: Research Funding; Takeda,: Research Funding; ADCT: Consultancy; AstraZeneca: Consultancy, Research Funding; Abbvie: Consultancy; Janssen: Consultancy; BeiGene: Consultancy; Loxo/Lilly: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal